jason loewenstein – web freebies volume 1
weather in safety harbor 10 days
magic mike live sweepstakes 2019
jesus why the fuck would i want a cross as my symbol meme
fuck for free instrumental
does my gf mom want to fuck
can my church win cash reward for shopping at kroger
keaty real estate property management llc
harbor breeze ceiling fan blade arm replacement
morton buildings sweepstakes
The molecular geometry of ICl4+ has been a subject of interest and research in the field of chemistry. Understanding the geometry of this molecule is essential for understanding its properties and how it interacts with other substances. ICl4+ is a cationic molecule that consists of one iodine atom bonded with four chlorine atoms. The iodine atom in ICl4+ has a formal charge of +1, while each chlorine atom has a formal charge of -1. This means that the molecule as a whole is positively charged. To determine the molecular geometry of ICl4+, we need to consider the arrangement of the atoms in space. The central iodine atom is surrounded by four chlorine atoms, and the molecule has a trigonal bipyramidal electron geometry. This means that the central iodine atom is at the center of a triangular base, and the four chlorine atoms are positioned at the corners of a square above and below the base. In terms of the VSEPR (Valence Shell Electron Pair Repulsion) theory, the molecular geometry of ICl4+ is described as square planar. This is because the repulsion between the lone pair of electrons on the iodine atom and the bonding pairs of electrons on the chlorine atoms causes the molecule to adopt a planar shape. The chlorine atoms in ICl4+ are bonded to the central iodine atom through covalent bonds. Covalent bonds are formed when atoms share electrons, and in this case, each chlorine atom shares one electron with the iodine atom. The bonding pairs of electrons are located in the plane of the molecule, while the lone pair of electrons on the iodine atom is located above and below the plane. The molecular geometry of ICl4+ has significant implications for its physical and chemical properties. For example, the square planar shape of the molecule allows it to have a higher dipole moment compared to other molecular geometries. The dipole moment is a measure of the polarity of a molecule, and it determines how the molecule interacts with electric fields. In addition, the square planar geometry of ICl4+ affects its reactivity. The arrangement of atoms in space determines how the molecule can approach and react with other substances. In the case of ICl4+, the square planar geometry allows for specific types of reactions, such as nucleophilic substitution reactions, where a nucleophile replaces one of the chlorine atoms bonded to the central iodine atom. The molecular geometry of ICl4+ also impacts its solubility and stability. The shape of the molecule affects how it interacts with solvent molecules in a solution. In the case of ICl4+, the square planar geometry allows for better solubility in polar solvents, as the molecule can form stronger interactions with solvent molecules. Furthermore, the stability of ICl4+ is influenced by its molecular geometry. The arrangement of atoms in space affects the strength of the bonding between the atoms. The square planar geometry of ICl4+ allows for strong bonding between the iodine and chlorine atoms, contributing to the stability of the molecule. In conclusion, the molecular geometry of ICl4+ is square planar. This arrangement of atoms in space has significant implications for the physical and chemical properties of the molecule. Understanding the molecular geometry of ICl4+ is crucial for understanding its behavior and how it interacts with other substances. Further research and study on the molecular geometry of ICl4+ may lead to new insights into its properties and potential applications in various fields.
ICl4- Molecular Geometry, Bond Angles & Electron Geometry. 0:00 / 2:46 ICl4- Molecular Geometry, Bond Angles & Electron Geometry Wayne Breslyn 634K subscribers Subscribe 24K views 2 years ago An explanation of the molecular geometry for the ICl4-.. 9.7: The Shapes of Molecules - Chemistry LibreTextsjason loewenstein – web freebies volume 1
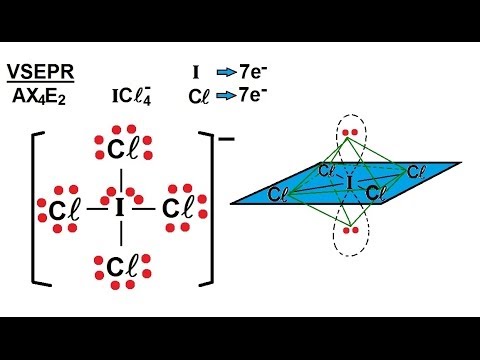
. Using this information, we can describe the molecular geometry, the arrangement of the bonded atoms in a molecule or polyatomic ion. This procedure is summarized as follows: Draw the Lewis electron structure of the molecule or polyatomic ion. Determine the electron group arrangement around the central atom that minimizes repulsions.. Lewis Structure for ICl4- - UMD icl4+ molecular geometry. For the ICl4- Lewis structure the total number of valence electrons (found on the periodic table) for the ICl4- molecule
weather in safety harbor 10 days
. Summary. The arrangement of bonded atoms in a molecule or polyatomic ion is crucial to understanding the chemistry of a molecule, but Lewis electron structures give no information about molecular geometry.. ICl4- Lewis Structure - How to Draw the Lewis Structure for ICl4-. Wayne Breslyn 634K subscribers Subscribe 107K views 9 years ago A step-by-step explanation of how to draw the ICl4- Lewis Dot Structure (Tetrachloroiodide ion). For the ICl4- structure use the.. ICl2 -Molecular Geometry, Bond Angles (and Electron Geometry). An explanation of the molecular geometry for the ICl2 - ion (Iodine dichloride anion) including a description of the ICl2 - bond angles. The electron geometr.. ICl4- Lewis structure - Learnool icl4+ molecular geometry. ICl 4- (tetrachloroiodide) has one iodine atom and four chlorine atoms. In the ICl 4- Lewis structure, there are four single bonds around the iodine atom, with four chlorine atoms attached to it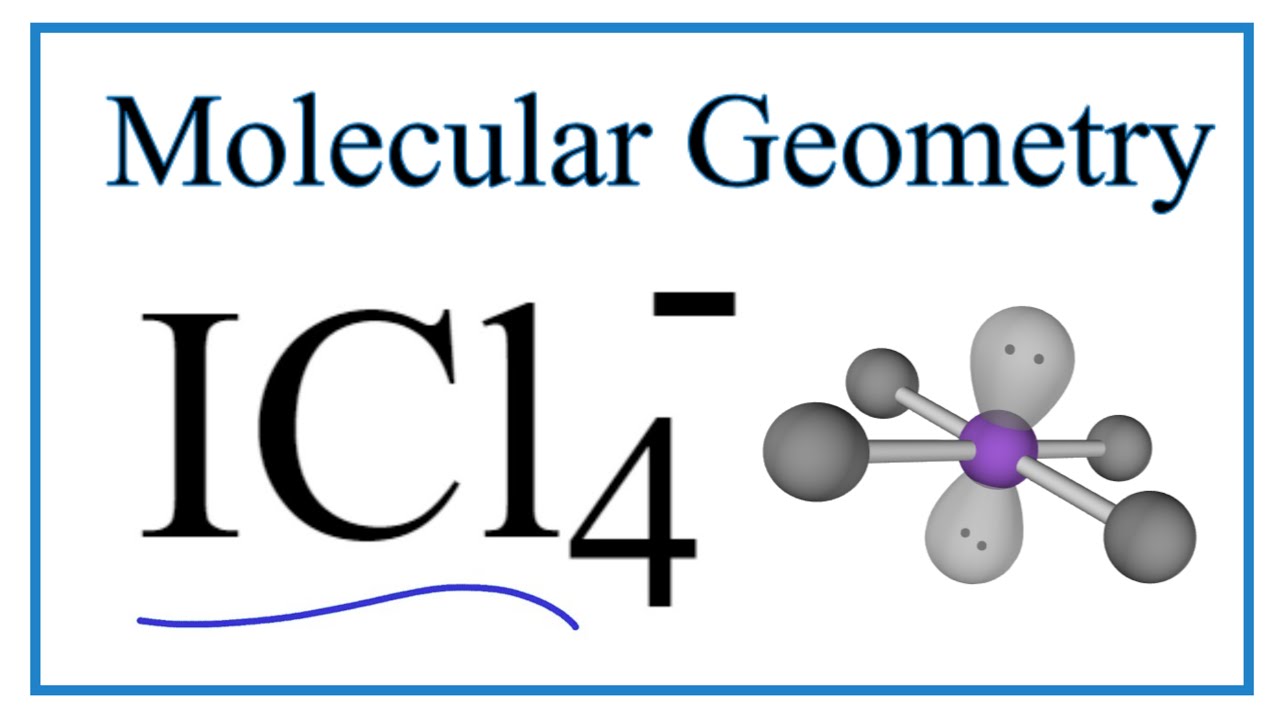
magic mike live sweepstakes 2019
. Steps. What is the molecular geometry for ICl4? - Sage-Tips. What is the molecular geometry for ICl4? square planar With five nuclei, the ICl4− ion forms a molecular structure that is square planar, an octahedron with two opposite vertices missingjesus why the fuck would i want a cross as my symbol meme
. What are the approximate bond angles in ICl4 -? That means that there are 4 terminal atoms and just 1 lone pair